All API Impurities and Reference Standards
Category starting from letter:
Displaying 1 to 7 ( of 7 products )
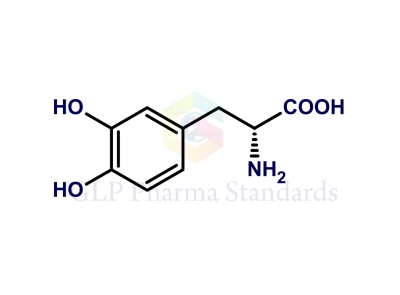
Levodopa EP Impurity A
Levodopa EP Impurity A
| Catalogue No : | GL-L0702 |
| Common name: | Levodopa EP Impurity A; Levodopa USP Related Compound A; 2-Hydroxy Levodopa |
| CAS : | 27244-64-0 |
| Mol. weight | 213.19 |
Levodopa EP Impurity B
Levodopa EP Impurity B
| Catalogue No : | GL-L0703 |
| Common name: | Levodopa EP Impurity B; L-Tyrosine |
| CAS : | 60-18-4 |
| Mol. weight | 181.19 |
Levodopa EP Impurity C
Levodopa EP Impurity C
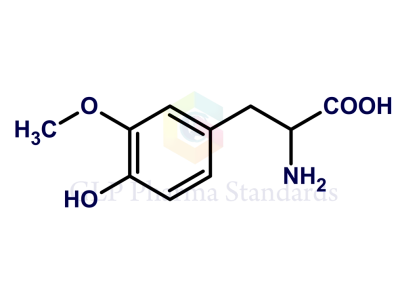
| Catalogue No : | GL-L0704 |
| Common name: | Levodopa EP Impurity C; Levodopa USP Related Compound B; 3-Methoxy-DL-tyrosine |
| CAS : | 7636-26-2 |
| Mol. weight | 211.21 |
Levodopa EP Impurity D
Levodopa EP Impurity D
| Catalogue No : | GL-L0705 |
| Common name: | Levodopa EP Impurity D; D-Dopa |
| CAS : | 5796-17-8 |
| Mol. weight | 197.19 |
Levodopa sulfate
Levodopa sulfate
| Catalogue No : | GL-L0706 |
| Common name: | Levodopa sulfate |
| CAS : | N. A. |
| Mol. weight | 277.25 |
3-Acetyl-L-tyrosine Hydrochloride
3-Acetyl-L-tyrosine Hydrochloride
| Catalogue No : | GL-L0708 |
| Common name: | 3-Acetyl-L-tyrosine Hydrochloride |
| CAS : | 32404-28-7(HCl Salt); 32483-30-0(Freebase) |
| Mol. weight | 259.69 |